lewis structure sodium chloride|6.11A: Structure : Tuguegarao Ene 11, 2023 — Thus the chlorine gains an electron from the sodium atom. The Lewis Structure of this reaction is (here we will consider one chlorine atom, rather than Cl 2) is: The arrow indicates the transfer of the electron from sodium to chlorine to form the Na + metal ion and the Cl-chloride ion
Tag: National Bureau of Investigation (NBI) SEMRO XI. Local News. Content Creator ‘Brader’, nasikop sa NBI XI. Bombo Angie Villones Chagas-August 17, 2024 | 4:05 PM.
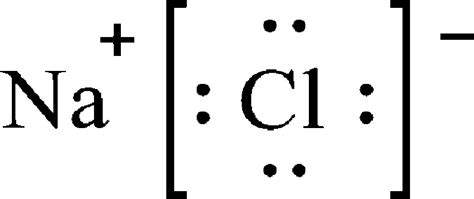
lewis structure sodium chloride,Okt 14, 2020 — A step-by-step explanation of how to draw the NaCl Lewis Dot Structure (Sodium chloride).For NaCl we have an ionic compound and we need to take that into acc.
The Lewis Structure for the Salt NaCl depicts two ions with complete octets in their outer shells of electrons. As a result, there is a positive charge on sodium (due to one lost electron) and a negative charge on .
Hul 26, 2019 — How to Draw the Lewis Structure of NaCl (sodium chloride, ionic) chemistNATE. 266K subscribers. Subscribed. 1.1K. 101K views 4 years ago Lewis Structures. Check me out:.Nob 19, 2013 — For the NaCl Lewis structure, calculate the total number of valence electrons for the NaCl molecule. Since Na is a metal and Cl is a non-metal we have an ionic compound for the Lewis dot.
It is extracted from the mineral form halite or evaporation of seawater. The structure of NaCl is formed by repeating the face centered cubic unit cell. It has 1:1 stoichiometry ratio of Na:Cl with a molar mass of 58.4 g/mol. .Ene 11, 2023 — Thus the chlorine gains an electron from the sodium atom. The Lewis Structure of this reaction is (here we will consider one chlorine atom, rather than Cl 2) is: The arrow indicates the transfer of the electron from sodium to chlorine to form the Na + metal ion and the Cl-chloride ion
This page contains materials for the session on Lewis structures. It features a 1-hour lecture video, and also presents the prerequisites, learning objectives, reading assignment, lecture slides, homework with solutions, and resources for further study. . sodium (Na), chloride (Cl), nitrogen (N), oxygen (O), lithium (Li), beryllium (Be .
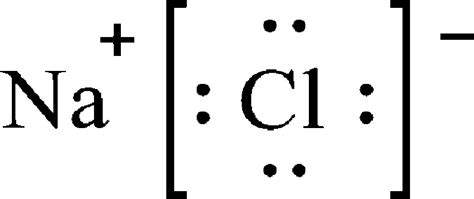
Okt 11, 2023 — The total valence electron is available for drawing the Sodium chloride (NaCl) lewis structure is 8. NaCl is a face-centered cubic unit cell that has four cations and four anions. In the NaCl lewis dot structure, the sodium atom completes its octet by transferring one electron to the chlorine atom.
lewis structure sodium chlorideThus the chlorine gains an electron from the sodium atom. The Lewis Structure of this reaction is (here we will consider one chlorine atom, rather than Cl 2) is: The arrow indicates the transfer of the electron from sodium to chlorine to form the Na + metal ion and the Cl-chloride ion
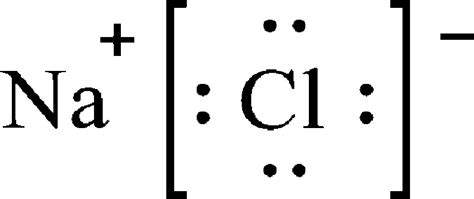
May 6, 2024 — What is the Lewis Structure of Sodium chloride? Discover the Lewis Structure of Sodium chloride and delve into its molecular geometry, bonding, and applications. Learn how to draw NaCl's Lewis structure and understand its nonpolar nature and ionic lattice structure. Hadley1 MIN READMay 6, 2024May 6, 2024 — What is the Lewis Structure of Sodium chloride? Discover the Lewis Structure of Sodium chloride and delve into its molecular geometry, bonding, and applications. Learn how to draw NaCl's Lewis structure and understand its nonpolar nature and ionic lattice structure. Hadley1 MIN READMay 6, 2024
If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.The image below shows how sodium and chlorine bond to form the compound sodium chloride. Unlike sodium metal, the resulting compound is not explosive and is less corrosive than chlorine gas. . (+2 - 1 - 1 = 0). This Lewis structure requires two chloride anions per one magnesium cation to produce a stable compound. Contributors and .Sodium chloride / ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d /, [8] commonly known as edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chlorine ions. It is transparent or translucent, brittle, hygroscopic, and occurs as the mineral halite.In its edible form, it is commonly used as a condiment and food preservative.Large .Hun 26, 2023 — The image below shows how sodium and chlorine bond to form the compound sodium chloride. Unlike a sodium atom, the resulting compound is not explosive and less corrosive than chlorine. . Cation .
Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript. Other names: Salt Permanent link for this species. Use this link for bookmarking this species for future reference. Information on this page: Notes; Other data available: Gas phase .lewis structure sodium chloride 6.11A: Structure For example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons: The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between the atoms). A dash (or line) is sometimes used to indicate a shared pair .
Hun 20, 2023 — Example \(\PageIndex{1}\): Lewis Structures. Solution; Lewis used simple diagrams (now called Lewis diagrams) to keep track of how many electrons were present in the outermost, or valence, shell of a given atom.The kernel of the atom, i.e., the nucleus together with the inner electrons, is represented by the chemical symbol, and only the .Ago 10, 2022 — A neutral sodium atom is likely to achieve an octet in its outermost shell by losing its one valence electron. \[\ce{Na \rightarrow Na^{+} + e^{-}} \nonumber \] The cation produced in this way, Na +, is called the sodium ion to distinguish it from the element. The outermost shell of the sodium ion is the second electron shell, which has eight .
Sodium chloride is the chemical name of NaCl. The NaCl Molecular Weight (Sodium Chloride) is 58.44 g/mol. Visit BYJU'S to understand the properties, structure, and uses of Sodium Chloride (NaCl) explained by India's best teachers.
Mar 27, 2023 — NaCl is a chemical formula for sodium chloride. And to help you understand the Lewis Structure of this molecule, we are going to share our step-by-step metho.6.11A: Structure Lewis structures (also known as Lewis dot diagrams, electron dot diagrams,"Lewis Dot formula" Lewis dot structures, and electron dot structures) are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well .
lewis structure sodium chloride|6.11A: Structure
PH0 · What is the Lewis Structure of Sodium chloride?
PH1 · NaCl Lewis Structure: How to draw the Lewis Dot Structure for
PH2 · NaCl Lewis Structure: How to draw the Lewis Dot
PH3 · Lewis Structure of NaCl
PH4 · Lewis Dot Structure for Sodium Chloride
PH5 · How to Draw the Lewis Structure of NaCl (sodium chloride, ionic)
PH6 · How to Draw the Lewis Dot Structure for NaCl: Sodium chloride
PH7 · 6.11A: Structure